The Follicle-Stimulating Hormone (FSH) Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of Follicle-stimulating hormone (FSH) level in urine to evaluate the onset of menopause in women.A multi-center clinical evaluation was conducted comparing results obtained using the Follicle-Stimulating Hormone (FSH) Rapid Test to another commercially available urine membrane FSH test. The study included 200 urine specimens:both assays identifified 120 negative and 78 positive results. The results demonstrated 99% accuracy of the FSH Rapid Test when compared to the other urine membrane FSH test.
The testing for FSH can help determine whether a woman is in the perimenopause stage. If a woman knows she is perimenopausal, she can take the appropriate steps to keep her body healthy and avoid the health risks associated with menopause,which include osteoporosis, increased blood pressure and cholesterol, and increased risk of heart disease.
The Follicle-Stimulating Hormone (FSH) Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of Follicle-stimulating ho rmone (FSH) level in urine to evaluate the onset of menopause in women.
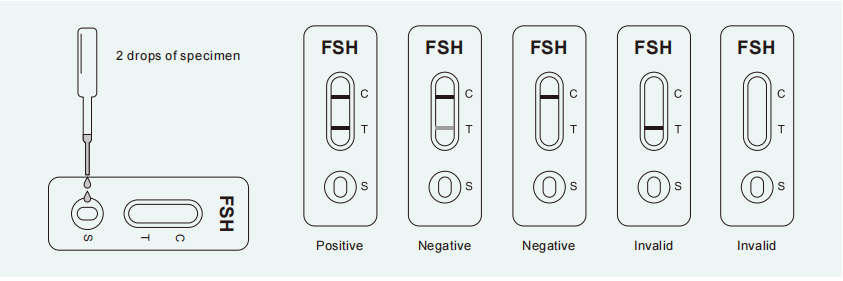
POSITIVE: Two lines are visible and the test line (T) is the same as or darker than the control line (C). A positive result indicates that FSH levels are higher than normal and the subject you may be experiencing
perimenopause.
NEGATIVE: Two lines are visible, but the test line (T) is lighter than the control line (C), or there is no test line. A negative result indicates that the subject is probably not experiencing perimenopause in this cycle.
INVALID: Control line (C) fails to appear. Insufficient specimen volume or incorrect test performance are the most likely reasons for an invalid result. Review the procedure and repeat with a new test. If the problem persists,discontinue using the test kit immediately and contact your local distributor.
| Product name | Follicle-Stimulating Hormone (FSH) Rapid Test |
| Specimen | Urine |
| Format | Cassette |
| Pack/box | 25T |
| Expiration date | 24months |
| Storage temperature | 2-30℃ |
| Certificate | CE |

To monitor your health status anytime, anywhere and achieve self-inspection and self-testing.