Malaria antigen test using the technical principle of the double antibody sandwich method. It is a rapid in vitro diagnostic reagent for the qualitative detection of Plasmodium antigens in human whole blood specimens. It not only detects whether a person is infected with malaria within 10 minutes, but also determines whether the infection is Plasmodium falciparum or Plasmodium falciparum. It is simple to use and easy to interpret and has high sensitivity, specificity and accuracy.
Malaria is an infectious disease caused by plasmodium parasites in the human body. Infected by malaria mosquito bites or transfusion of the blood of a person carrying malaria parasites. Difffferent malaria parasites cause vivax,falciparum and ovarian malaria respectively. Malaria is mainly manifested as periodic and regular attacks, with body chills, fever, and hyperhidrosis. After a long period of repeated attacks, it can cause pelvic blood and splenomegaly. Children have a high morbidity rate, mostly in summer and autumn. The mortality rate of falciparum malaria is extremely high. Therefore, it is very important to reduce the time to eliminate through detection.
This product is used for qualitative detection of Plasmodium falciparum antigen and Plasmodium vivax antigen in venous blood/fingertip blood of people with symptoms and signs of malaria.
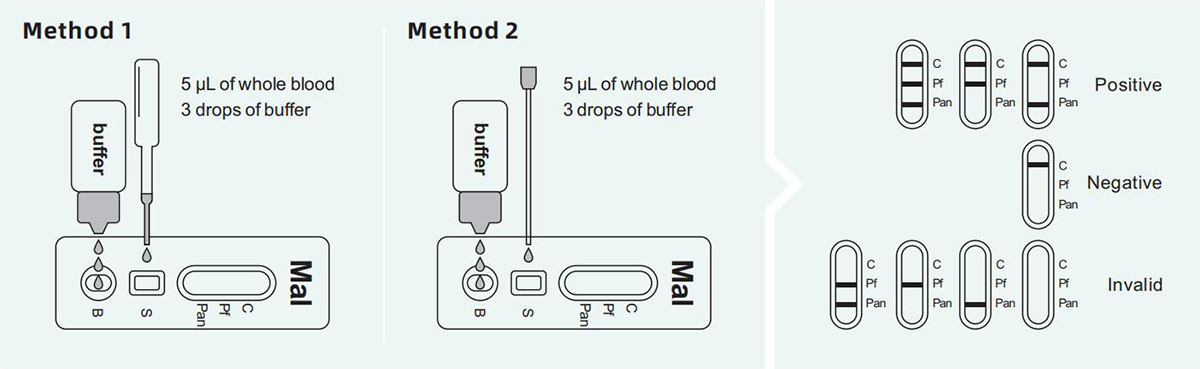
P.f. positive (+): One color line should be in the control area (C), and the other color line should be in the detection area P.f.. The result is positive for Malaria P.f..
Pan positive (+): One color line should be in the control area (C), and the other color line should be in the detection area Pan. The result The result is positive for Malaria Pan.
P.f. and Pan positive (+): The control area (C) should have one color line, and the detection line area should have two color lines for P.f. and Pan. The result was positive for Malaria P.f. and Pan.
Negative (-): Only a purple-red band appears in the quality control area (C). There is no purple-red band in the detection area (P.f. 、Pan).
Invalid: There is no purple-red band in the quality control area (C).
| Product name | Malaria P.f./Pan Antigen Rapid Test Cassette (Colloidal Gold Method) | |
| Specification | 25pcs/box | |
| Specimen | whole blood | |
| Expiration date | 24 months | |
| Storage temperature | 2-30℃ | |
| Sensitivity | P.f. | 99.90% |
| Pan | 98.00% | |
| Specificity | 99.90% | |
| Accuracy | 99.80% | |

To monitor your health status anytime, anywhere and achieve self-inspection and self-testing.