Used for qualitative in vitro detection of norovirus antigen type I /II in human fecal samples. Used to assist in the diagnosis of norovirus infection. Norovirus is one of the main pathogens causing non-bacterial acute gastroenteritis at present. This virus is highly infectious, mainly transmitted through contaminated water, food and other ways, contact transmission or aerosol formed by pollutants. It is the second pathogen that causes viral diarrhea in children after rotavirus, and it breaks out collectively in crowded places.
Norovirus belongs to the caliciviridae family. The particles are 20-hedral symmetrical, 27~35nm in diameter, and have no capsule membrane. Norovirus is one of the main pathogens causing non-bacterial acute gastroenteritis at present. This virus is highly infectious, mainly transmitted through contaminated water, food and other ways, contact transmission or aerosol formed by pollutants. It is the second pathogen that causes viral diarrhea in children after rotavirus, and it breaks out collectively in crowded places. Norovirus is mainly divided into five genomes (GI, GII, GIII, GIV and GV), which mainly infect people with GI, GII and GIV, among which the norovirus of GII genome is the most common virus strain worldwide. The main clinical or laboratory diagnostic methods for norovirus infection include electron microscopy, molecular biology and immunological detection.
Used for qualitative in vitro detection of norovirus antigen type I /II in human fecal samples. Used to assist in the diagnosis of norovirus infection.
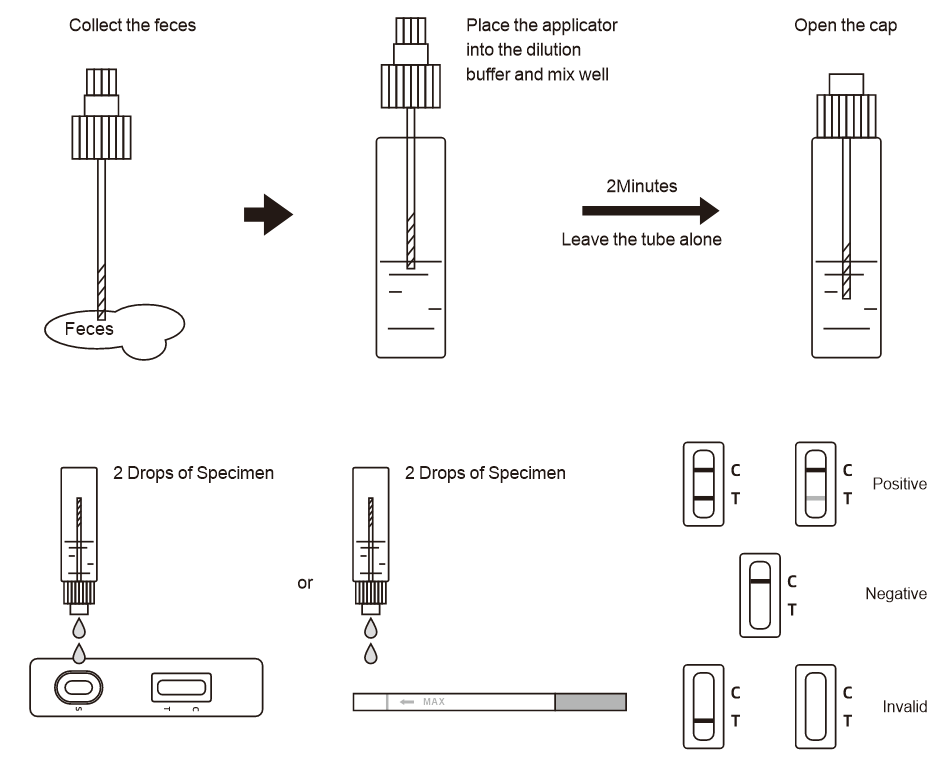
Positive (+): Two purple-red bands appear. One is located in the detection area (T), and the other is located in the quality control area (C). The positive results showed that the samples contained the myocardial marker.
Negative (-): Only a purple-red band appears in the quality control area (C). No purple-red bands appeared in the detection areas (T). Negative results indicate that the specimen does not contain the myocardial markers.
INVALID: If the control line (C) is not visible within the result window after performing the test, the result is considered invalid. Some causes of invalid results are because of not following the directions correctly or the test may have deteriorated beyond the expiration date. At this time, you should read the instructions carefully again, and use a new reagent to test again. If the problem still exists, stop using this batch immediately and contact the local supplier.

To monitor your health status anytime, anywhere and achieve self-inspection and self-testing.