The Respiratory Syncytial Virus(RSV) Rapid Test is an in vitro diagnostic test for the qualitative Detection of Respiratory Syncytial Virus antigens (viral fusion protein) in nasal swab, throat Swab or nasopharyngeal swab, using the rapid immuno-chromatographic method. It is intended to aid in the rapid differential diagnosis of Respiratory Syncytial Virus viral infections.
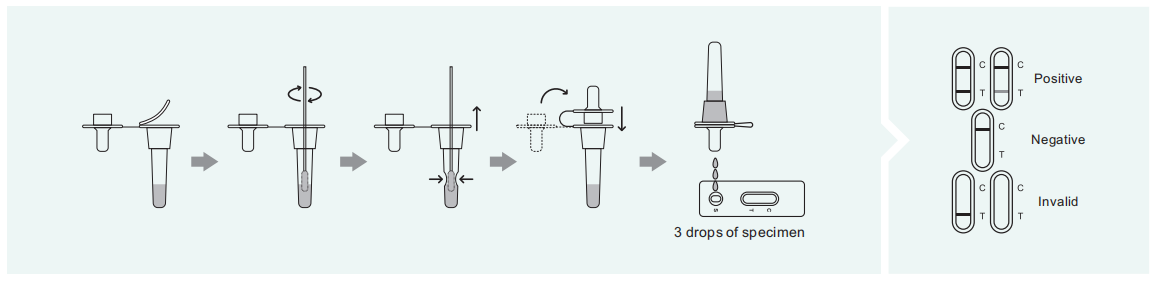
POSITIVE: Two lines appear. One colored line appears in the control region©, and another Apparent colored line in the test region(T). The shade of color may vary, but it should be Considered positive whenever there is even faint line.
NEGATIVE: Only one colored line appears in the control region(C). No line in the test region(T). The negative result in dictates that there are no Respiratory Syncytial Virus particles in the sample or the number of viral particles is below the detectable range.
INVALID: There is no purple-red band in the quality control area (C).In any case, it should be retested.
| Product name | RespiratorySyncytial Virus(RSV) Rapid Test |
| Specimen | Swab |
| Format | Cassette |
| Pack/box | 25T |
| Expiration date | 24months |
| Storage temperature | 2-30℃ |
| Certificate | CE |

To monitor your health status anytime, anywhere and achieve self-inspection and self-testing.