Syphilis Rapid Test is based on the technical principle of the double sandwich method. The test is simple to perform and can be done in one step. Comprehensive specimen coverage, whole blood, serum and plasma samples can be tested. The test is rapid and results can be read in 15 minutes. Stable and can be stored at room temperature for up to 24 months.
Syphilis is a chronic sexually transmitted disease caused by Treponema pallidum. When a pathogen invades the human body, it can stimulate the human immune system to produce specific antibodies against Treponema pallidum. Under natural conditions, Treponema pallidum only infects humans, so syphilis patients are the only source of infection. Treponema pallidum antibody is a specific antibody that appears after a person is infected with Treponema pallidum.
Used for in vitro qualitative detection of Treponema pallidum antibodies in whole blood, serum and plasma.
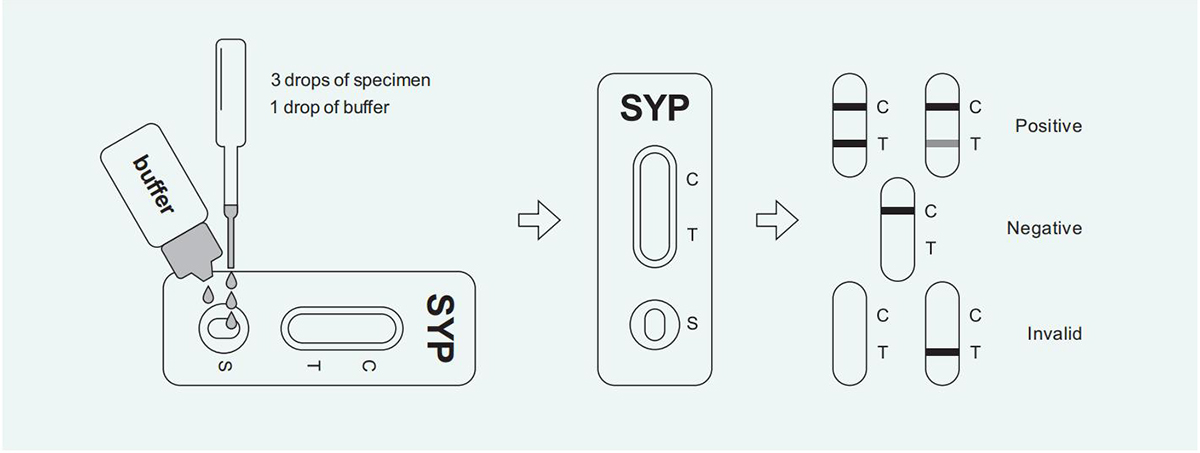
Positive (+): Two red lines appear. One line should be in the detection area (T), and another line should be in the quality control area (C).
Negative (-): Only one red line appears in the quality control area (C). There is no line appears in the detection area (T).
Invalid: There is no line appears in the quality control area (C).
| Product name | Syphilis Rapid Test Cassette | |
| Specification | WB/S/P | WB/S/P |
| Format | Cassette | Strip |
| Pack/Box | 25T | 50T |
| Expiration date | 24 months | |
| Storage temperature | 2-30℃ | |
| Certifificate |  | |

To monitor your health status anytime, anywhere and achieve self-inspection and self-testing.